AlexWilliamsGloves
Well-Known Member
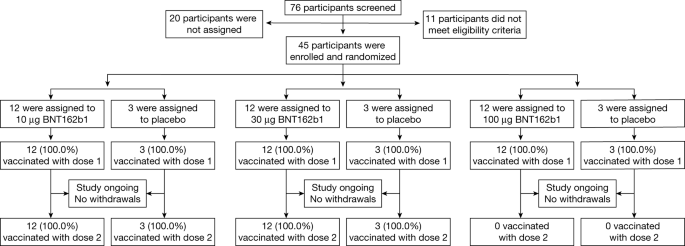
Posted this in another thread, but thought some might be interested in what i learned....the new vaccine isnt like other typical vaccinations.....ie, it DOESNT inject a small amount of the virus into you. So those who are afraid of contracting covid from the vaccination are incorrect...

 factcheckni.org
factcheckni.org
What is this COVID-19 mRNA vaccine?
The Centers for Disease Control and Prevention described this COVID-19 vaccine as a messenger RNA vaccine — or a mRNA vaccine.
This type of vaccine does not put a weakened or inactivated germ into our bodies. Rather, mRNA teaches our cells how to make a protein — or even just a piece of a protein — that triggers an immune response inside our bodies. COVID-19 mRNA vaccines give instructions for our cells to make a harmless piece of what is called the “spike protein”. This “spike protein” is found on the surface of the virus that causes COVID-19. The immune response to this — our body’s production of antibodies — is what protects us from getting infected if the real virus enters our bodies.
It has been explained that a major advantage of RNA vaccines is that RNA can be produced in the laboratory from a DNA template using readily available materials, less expensively and faster than conventional vaccine production.

The COVID-19 mRNA vaccine: What is it and how has it been developed so quickly? - FactCheckNI
The Pfizer/BioNTech coronavirus vaccine has been approved for use in the UK from the week commencing 6 December 2020. This means that the UK has become the first country in the world to approve the vaccine for widespread use. MHRA, the British regulator, says the jab, which offers up to 95%...
What is this COVID-19 mRNA vaccine?
The Centers for Disease Control and Prevention described this COVID-19 vaccine as a messenger RNA vaccine — or a mRNA vaccine.
This type of vaccine does not put a weakened or inactivated germ into our bodies. Rather, mRNA teaches our cells how to make a protein — or even just a piece of a protein — that triggers an immune response inside our bodies. COVID-19 mRNA vaccines give instructions for our cells to make a harmless piece of what is called the “spike protein”. This “spike protein” is found on the surface of the virus that causes COVID-19. The immune response to this — our body’s production of antibodies — is what protects us from getting infected if the real virus enters our bodies.
It has been explained that a major advantage of RNA vaccines is that RNA can be produced in the laboratory from a DNA template using readily available materials, less expensively and faster than conventional vaccine production.