roubaixtuesday
Well-Known Member
- Joined
- 14 Dec 2019
- Messages
- 5,734
- Team supported
- City
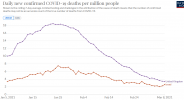
Wrong, they presented viable scenarios to the public on the eve of the November lockdown where daily deaths hit 2,000 - 4,000.
Actually I was correct, you're referencing a different piece of work.
But what's your point? They presented scenarios with a huge surge in deaths and we got one.
They're now saying a sudden opening up brings a likelihood of another surge.
By all means provide a link to the modelling if you want to discuss the details.